Batteries consist of multiple voltaic cells or one cell connected to form a unit. This unit is called a battery. Each battery contains two metal plates and a chemical that consists of positive and negative ions. The two metal plates form the electrode, and the chemical present in the battery is the electrolyte. A battery converts the chemical energy inside the cell into electrical energy, which helps us power electronic devices. It provides electricity to laptops, mobile phones and even clocks. To understand the chemistry of Batteries further, we will first study the two main types of batteries–
Read about Technical Analysis of Stocks for Beginners: 1 Complete Guide on Technical Analysis Of Stocks for Beginners
Primary Battery
A type of battery in which the chemical reaction occurs only once. This property limits primary batteries to recharge like other batteries. Flashlights, remote controls, televisions, and smoke detectors use primary batteries. The most common example of a primary battery is the dry cell called the Leclanche cell (Zinc-carbon battery).
Working of primary batteries | Chemistry of Batteries
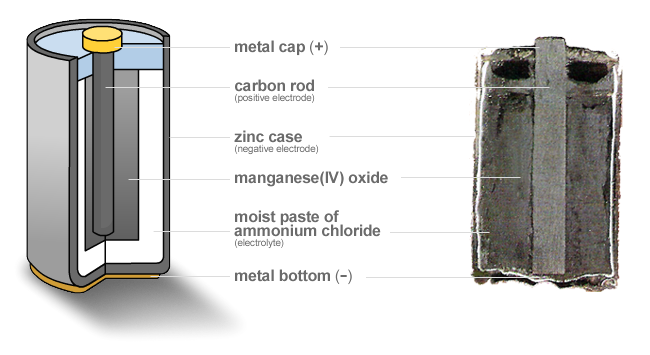
- Zinc-carbon battery.
There are two components of zinc-carbon batteries: the zinc shell and the carbon rod. It is the first mass-produced battery used as a source of electric supply in appliances.
The zinc shell acts as the anode and functions as a container. And the carbon rod acts as the cathode, surrounded by a layer of powdered manganese dioxide. The space between the cathode and anode consists of ammonium chloride(NH4CL) and zinc chloride(ZnCl2).
The zinc atoms oxidize on the anode and combine with the ammonium ions to form ZNNH304, while manganese dioxide undergoes reduction on the cathode.
Reaction:
Zn(s) → Zn2+(aq) + 2e–
2e– + 2NH4+(aq) → 2NH3(g) + H2(g)
Overall reaction:
Zn(s) + 2NH4+(aq) + 2MnO2(s) → [ Zn(NH3)2]2+(aq) + Mn2O3(s) + H20(l)
Because these are oxidation-reduction reactions that occur, they are called half-reactions. The reaction which takes place in the zinc-carbon dry cell produces 1.5V.
The zinc-carbon battery is used in appliances that require low to medium electric supply.
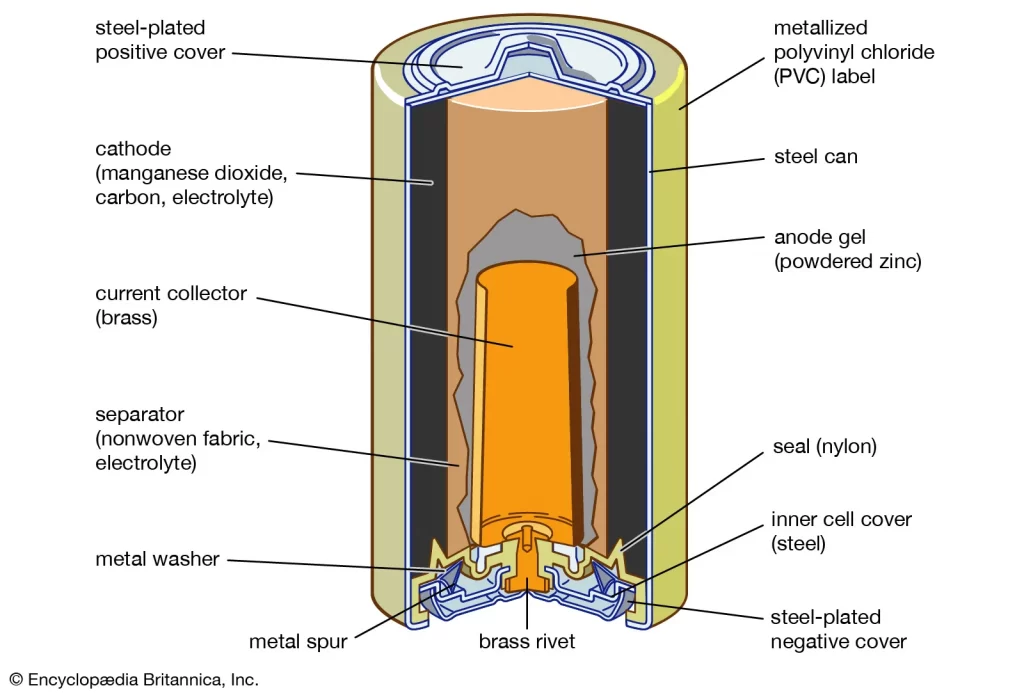
- Alkaline Battery
Alkaline batteries use zinc at the anode and manganese dioxide at the cathode. The battery uses an alkaline chemical called potassium hydroxide. In the alkaline battery oxidation-reduction reaction, the zinc at the anode releases electrons to form Zinc oxide. These electrons get transferred to the cathode. Hence, manganese dioxide gets reduced.
Reactions
Zn(s) + 2OH–(aq) → ZnO(s) +H2O(l) + 2e–
2MnO2(s) + H2O(l) + 2e– → Mn2O3(s) + 2OH–(aq)
Overall reaction:
Zn(s) + 2MnO2(s) → ZnO(s) + MnO3(s)
Alkaline batteries have a longer shelf life than the Leclanche batteries, but they provide the same voltage of 1.5V.
Read and know about Information Literacy: Learn “What is information literacy? ” – in 5 easy steps
Secondary Battery:
In secondary batteries, the chemical reaction can be reversed and hence these batteries are rechargeable. The electrons discharged in these cells get restored, which helps the batteries recharge again once connected to the electric source. A secondary cell or battery is heavy and complex among all types of batteries. But it is still used in inverters and car batteries due to its cost-efficiency.
Working of secondary batteries
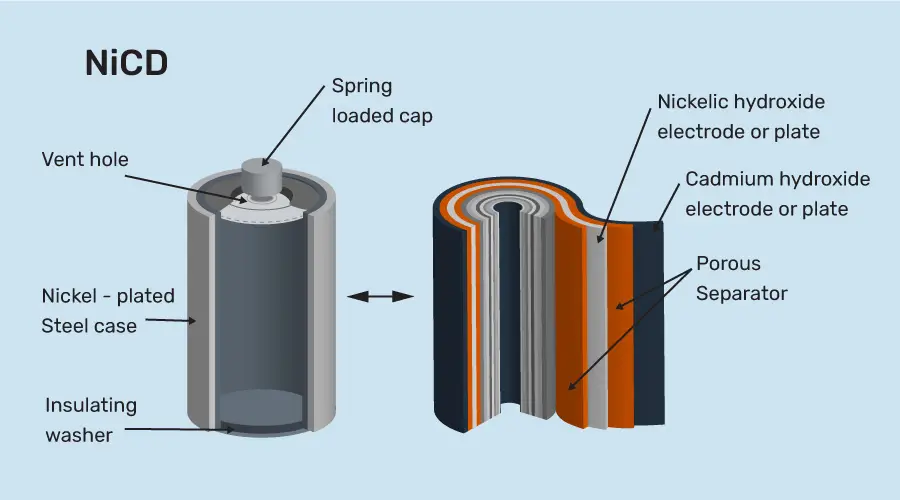
- Nickel-cadmium battery
As the name suggests, this battery contains nickel at the cathode and cadmium at the anode. The potassium hydroxide acts as the electrode in nickel-cadmium batteries. While charging, the nickel hydroxide present at the cathode forms nickel oxyhydroxide. And at the anode, cadmium hydroxide is formed from cadmium.
Reactions:
Cd(s) + 2OH–(aq) → Cd(OH)2(s) + 2e–
2NiO(OH)(s) + 2H2O(l) + 2e– → 2Ni(OH)2(s) + 2OH–(aq)
Overall reaction:
Cd(s) + 2 NiO(OH)(s) + 2H2O(l) → 2Ni(OH)2(s) + Cd(OH)2(s)
This battery gives a better performance than other alkaline batteries due to its jelly roll design. The overall voltage from the nickel-cadmium battery ranges from 1.2V to 1.25V. Nickel-cadmium batteries are present in computers and radio components.
- Lithium-ion batteries
While charging, the lithium ions in the batteries get transferred from anode to cathode. The movement of electrons gets reversed when the battery connects to a device. The lithium ions get released from the cathode to the anode. A separator in the lithium-ion battery functions as a block to the free flow of electrons.
Reactions:
Li(s) → Li+ + e–
Li+ + CoO2 + e– → LicoO2(s)
Overall reaction:
Li(s) + CoO2 → LiCoO2(s)
Lithium-ion batteries produce around 3.7V. They are present in cameras and tablets. Recently, lithium-ion batteries-are installed in electric cars on a large scale.
Conclusion
Batteries consist of voltaic cells that help in charging a device. Devices that use them include medical devices, vehicles, remote controls and submarines. Electric cars use lithium-ion and lead-acid batteries on a large scale. On the other hand, primary batteries are used- in household appliances. They are non-rechargeable and have an efficiency of less than 2%- due to which secondary batteries are preferable.
Secondary batteries have a high power density and a high discharge rate in comparison to primary batteries. They can be used, at low temperatures, unlike primary batteries. The initial cost of setting up a secondary battery is high, but it has efficient performance outputs.
The future use of cells and batteries is rising. Fuel cells use fuel in place of other chemicals in the electrochemical cell. This method is currently three times more efficient than the other methods.
Know the usage of Chemistry in Mechanical Engineering: What is mechanical engineering
FAQS (Frequently Asked Questions)
- Which batteries cannot recharge?
Primary batteries cannot recharge once used because the chemical reaction in primary batteries is not reversible. Primary batteries are present in household appliances like TV, radio, remote control and other electronic devices.
- What are the two types of batteries?
Primary and secondary batteries. Primary batteries are not rechargeable and have low efficiency. Secondary batteries are rechargeable. And are used as car batteries and also in electric vehicles.
- Why are secondary batteries better than primary batteries?
Secondary batteries have higher efficiency and recharge when connected to an electric supply. Most used secondary batteries and lithium-ion and nickel-cadmium batteries. Lithium-ion batteries work on jelly-rod designs.
- What are the chemicals used in batteries?
Chemicals such as zinc chloride, sodium chloride, potassium nitrate and magnesium hydroxide are present in electrochemical cells or batteries. These chemicals function as electrolytes in the batteries.
References
[1] Electrochemical Power Sources: Primary and Secondary Batteries, edited by M. Barak
[2] Introduction: Batteries and Fuel Cells, M. Stanley Whittingham Robert F. Savinell and Thomas Zawodzinski

 WhatsApp
WhatsApp